Introduction
Protein-protein interactions (PPIs) play a crucial role in regulating cellular functions and are enticing targets for drug discovery. However, modulating PPIs with small molecules presents significant challenges due to the intricate nature of these interactions. The Cyclic Ugi PPI Library offers a powerful tool for researchers to explore and exploit PPIs. In this blog post, we will explore the importance of PPIs, delve into the contents of the Cyclic Ugi PPI Library, and discuss their potential impact on targeted drug discovery.
Understanding Protein-Protein Interactions
Protein-protein interactions govern numerous biological processes, including signal transduction, enzymatic activity, and protein complex formation. Dysregulated or aberrant PPIs can lead to various diseases, making them attractive therapeutic targets. However, traditional drug discovery approaches often struggle to disrupt or modulate these intricate interactions. The development of small molecules that can specifically target and modulate PPIs represents an exciting frontier in drug discovery.
The Cyclic Ugi PPI Library
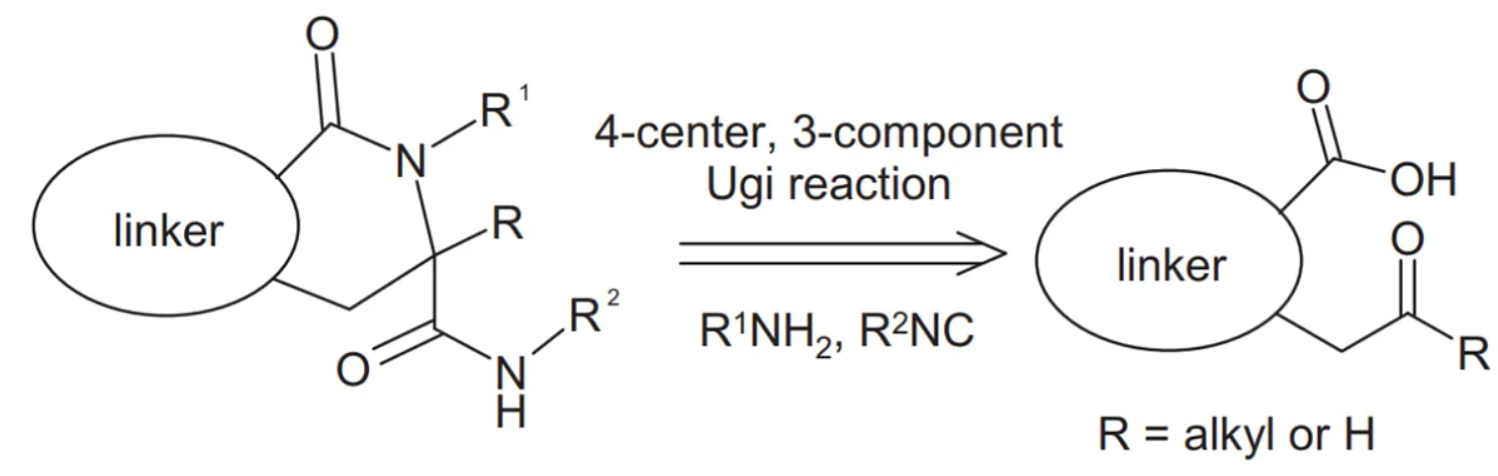
The Cyclic Ugi PPI Library is a curated collection of small molecules designed to disrupt or modulate PPIs. The library incorporates the Ugi multicomponent reaction, which combines four components in a single-step reaction, enabling the efficient synthesis of diverse and complex cyclic structures. By incorporating specific protein-binding motifs and pharmacophores, the library offers a vast array of cyclic small molecules with the potential to target and modulate specific PPIs. These molecules serve as valuable starting points for the development of targeted therapeutics.
Applications in Targeted Drug Discovery
The Cyclic Ugi PPI Library holds immense potential in the field of targeted drug discovery. By targeting specific PPIs, researchers can interrupt disease-promoting interactions or enhance beneficial interactions. These molecules can be designed to disrupt protein complexes involved in cancer, neurodegenerative disorders, viral infections, and autoimmune diseases. The library’s diverse chemical space provides researchers with ample opportunities to explore different targets, optimize compound characteristics, and identify lead candidates for further development.
Advantages and Challenges
The Cyclic Ugi PPI Library offers several advantages over traditional drug discovery approaches. Firstly, it provides a rapid and efficient method for generating diverse cyclic structures with drug-like properties. Additionally, the library’s cyclic nature allows for better shape complementarity and higher affinity towards specific binding sites. Moreover, the incorporation of protein-binding motifs increases the likelihood of achieving selectivity and specificity. However, like any drug discovery tool, challenges remain, such as predicting compound efficacy and off-target effects. Iterative optimization and the integration of computational modeling tools are essential for maximizing the success of the library in targeted drug discovery.
Future Perspectives
As the field of PPI-targeted drug discovery continues to advance, the Cyclic Ugi PPI Library holds great promise for accelerating therapeutic development. The ability to access a diverse library of cyclic small molecules offers researchers an advantage in tackling challenging PPI targets. Moreover, the iterative optimization process facilitated by the library allows for the identification of lead candidates for further exploration and refinement. With the advancement of computational tools and structural biology techniques, the potential for harnessing PPIs as effective therapeutic targets is expanding, and the Cyclic Ugi PPI Library stands at the forefront of this exciting frontier.
Conclusion
The Cyclic Ugi PPI Library represents a valuable resource for researchers seeking to unlock the potential of specific protein-protein interactions in targeted drug discovery. By providing diverse cyclic small molecules, the library enables researchers to explore and optimize compounds that modulate PPIs implicated in various diseases. As the understanding of PPIs and the library’s utility continues to expand, we can anticipate exciting advancements in the development of targeted therapeutics that disrupt critical interactions and improve patient outcomes.