Abstract
Epigenetic dysregulation plays a critical role in the onset and progression of various diseases, including cancer, inflammatory disorders, and neurological conditions. Bromodomain and extra-terminal (BET) proteins, which recognize acetylated lysine residues on histones, have emerged as promising therapeutic targets for modulating gene expression. The Bromodomain Modulators Library offers an extensive collection of small molecules designed to selectively inhibit or activate bromodomain-containing proteins. In this article, we explore the significance of bromodomain modulators, delve into the contents of the library, and discuss their potential applications in epigenetic-based therapies.
- Introduction
Epigenetic regulation involves the modification of gene expression patterns without altering the underlying DNA sequence. This regulatory mechanism plays a crucial role in development, cellular differentiation, and the maintenance of cellular identity. Perturbations in epigenetic processes can lead to the aberrant activation or silencing of genes, contributing to disease pathogenesis. Bromodomain-containing proteins, especially BET proteins, have emerged as key regulators of gene transcription and have garnered significant attention as therapeutic targets. - The Role of Bromodomain Proteins
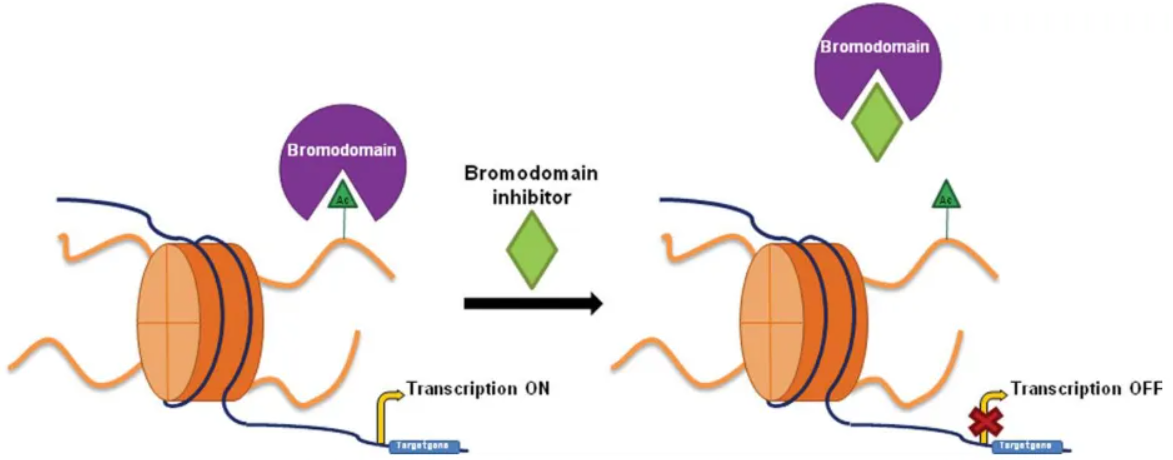
Bromodomain proteins are readers of epigenetic marks and facilitate transcriptional activation or repression. The BET family members, including BRD2, BRD3, BRD4, and BRDT, possess two bromodomains that interact with acetylated lysine residues on histones, promoting the recruitment of transcriptional machinery to gene promoters. Dysregulation of BET proteins has been implicated in numerous diseases, including cancer, inflammation, and heart disorders. Consequently, targeting these proteins with small molecules has emerged as a potential therapeutic strategy. - The Bromodomain Modulators Library
The Bromodomain Modulators Library comprises a diverse collection of small molecules that selectively interact with bromodomain-containing proteins. These molecules serve as modulators, either inhibiting the binding of the bromodomain to acetylated lysine residues or promoting their interaction. The library encompasses various chemical scaffolds, each offering unique features, selectivity profiles, and pharmacokinetic properties. This diversity empowers researchers to explore different bromodomain targets and optimize ligand-protein interactions for specific therapeutic applications. - Applications in Epigenetic-based Therapies
The Bromodomain Modulators Library holds immense promise in the field of epigenetic-based therapies. Inhibitors of bromodomain proteins, such as BET inhibitors, have demonstrated significant anti-cancer effects by suppressing key oncogenic drivers and reducing tumor growth1. These inhibitors have also shown efficacy in inflammation, cardiovascular diseases, and neurological disorders2. Conversely, bromodomain activators offer potential therapeutic opportunities by enhancing the expression of specific genes involved in tissue regeneration, cancer immunotherapy, or neurological repair3. Through their ability to modulate gene expression, bromodomain modulators hold great potential for developing targeted and personalized therapies. - Challenges and Future Directions
Despite the promise of bromodomain modulators, several challenges need to be addressed. BET protein inhibitors, although successful in preclinical and clinical trials, can exhibit resistance and undesired side effects. Achieving selectivity for specific bromodomain proteins and minimizing off-target effects remain major areas of focus. Moreover, the development of activators that can effectively modulate gene expression with high specificity and minimal adverse effects is an ongoing challenge. Continued research efforts, advanced methods for structure-based design, and identification of novel targets within the bromodomain family are instrumental in overcoming these challenges and realizing the full potential of bromodomain modulators.