Introduction:
Peptidomimetics have emerged as a promising class of molecules for drug discovery, offering enhanced properties compared to peptides. Among the diverse range of peptidomimetic libraries, those focused on beta-turn motifs have gained significant attention due to their importance in protein-protein interactions (PPIs) and their potential as therapeutic agents. This article explores the design, synthesis, and applications of peptidomimetics of beta-turn motifs libraries, highlighting their versatility as tools for drug discovery.
Understanding the Significance of Beta-Turn Motifs:
Beta-turns are secondary structures commonly found in proteins, characterized by a reverse turn in the backbone where hydrogen bonding between amino acid residues stabilizes the secondary structure. Beta-turns play crucial roles in protein folding, stability, and recognition processes. PPIs involving beta-turn motifs have been implicated in various biological pathways, including signaling cascades, immune responses, and diseases such as cancer and neurodegenerative disorders.
Design Principles and Approaches:
The design of peptidomimetics of beta-turn motifs libraries involves rational design and combinatorial chemistry techniques. Rational design utilizes structural information of beta-turn motifs from known protein-ligand complexes to generate compounds with improved binding affinity and specificity. Combinatorial chemistry employs a building-block approach to construct libraries with diverse peptidomimetic structures, allowing for a wider exploration of chemical space and potential for novel drug candidates.
Synthesis Strategies and Structural Diversity:
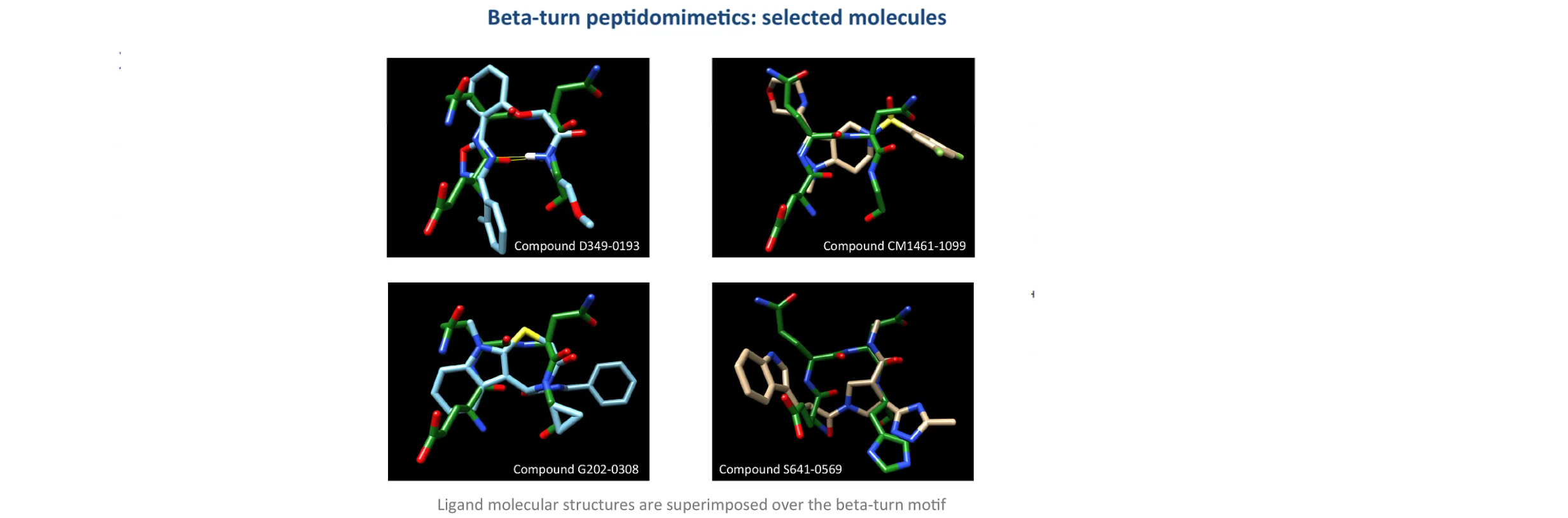
Peptidomimetics of beta-turn motifs libraries encompass a wide range of chemical scaffolds, including cyclic peptides, peptoids, constrained peptides, and non-peptide backbones. The synthesis of these libraries involves solid-phase synthesis, solution-phase synthesis, and diverse strategies to introduce modifications in the backbone and side chains. These modifications aim to mimic the topological and functional properties of beta-turn motifs, optimizing stability, bioavailability, and pharmacokinetic profiles.
Screening and Selection Methods:
The successful identification of lead compounds from a peptidomimetics library relies on efficient screening and selection methods. High-throughput screening assays, such as surface plasmon resonance and fluorescence polarization, enable the evaluation of compound binding to target proteins or receptor epitopes. Computational methods, including molecular docking and virtual screening, aid in the prediction of binding modes and the prioritization of compounds for further optimization.
Applications in Drug Discovery:
Peptidomimetics of beta-turn motifs libraries have shown great promise in drug discovery due to their ability to modulate PPIs associated with diseases. They have been applied in various therapeutic areas, such as cancer, infectious diseases, and neurological disorders. By targeting specific beta-turn motifs involved in PPIs, these libraries offer the potential to disrupt key molecular interactions, leading to the development of innovative therapeutic strategies.
Challenges and Future Perspectives:
The design and optimization of peptidomimetics of beta-turn motifs libraries pose several challenges. Achieving a balance between mimicking the desired structural features of beta-turn motifs and optimizing pharmacological properties can be complex. Additionally, the selection of suitable targets and clinical translation of these libraries remain important considerations. However, with advancements in computational biology, structural biology, and medicinal chemistry techniques, the potential for discovering potent drugs from peptidomimetics of beta-turn motifs libraries is promising.
Conclusion:
Peptidomimetics of beta-turn motifs libraries represent a valuable resource in drug discovery. By mimicking the structural and functional properties of beta-turn motifs, these libraries offer a toolbox for targeting PPIs involved in disease pathways. They provide versatility in chemical diversity, optimization of pharmacological properties, and potential as innovative therapeutic agents. Further research and development in this field hold great potential for the discovery of novel drugs to address unmet medical needs.
Suggested Reading:
Horne WS, Gellman SH. Peptide mimicry: cis-peptidomimetics and trans-peptidomimetics. Curr Opin Chem Biol. 2008 Aug;12(4):707-16.
Adams J, Keefe AD. Design, synthesis, and selection of DNA-encoded small-molecule libraries. Annu Rev Biochem. 2019 Jun 20;88:51-77.