Receptor protein tyrosine phosphatases (RPTPs) constitute a diverse family of cell surface receptors that play critical roles in cellular signaling pathways. Dysfunction or dysregulation of these receptors has been linked to various diseases, including cancer, neurodegenerative disorders, and autoimmune diseases. As a result, there is significant interest in developing small molecule drugs that selectively modulate the activity of RPTPs to combat these diseases. In this article, we will explore the importance of RPTPs as therapeutic targets and discuss small molecule drug design strategies for targeting these receptors.
Receptor Protein Tyrosine Phosphatases and Disease:
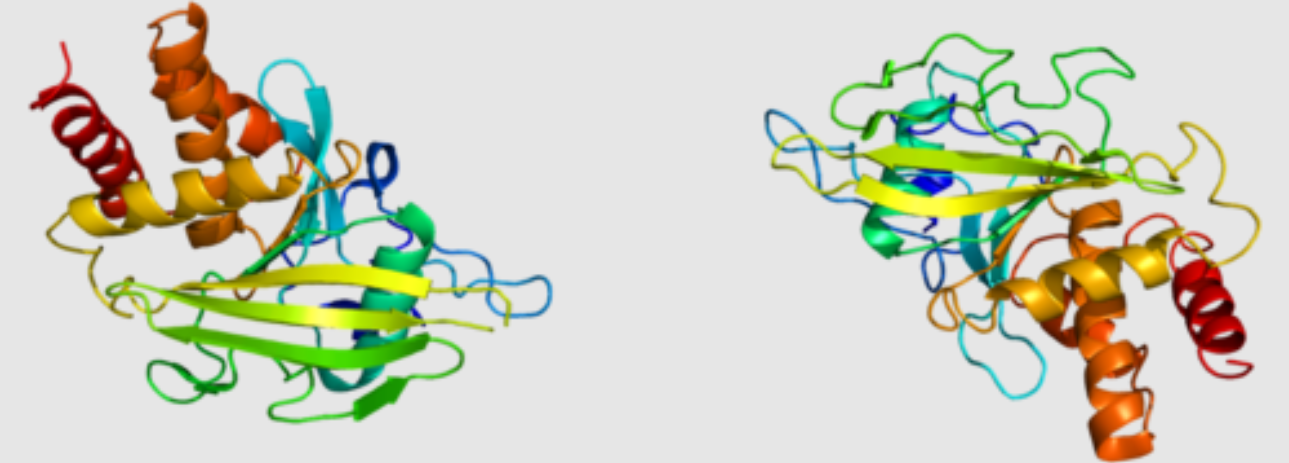
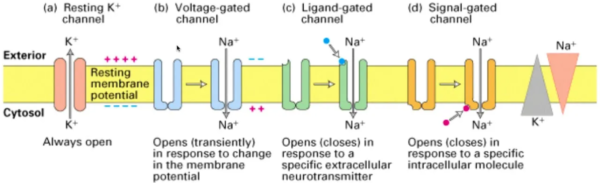
RPTPs are transmembrane proteins that possess a catalytic domain capable of dephosphorylating tyrosine residues on target proteins. They act as key regulators of cellular processes such as cell growth, differentiation, and adhesion. Dysregulation of RPTPs can disrupt normal cellular signaling, contributing to the development and progression of various diseases. For instance, alterations in RPTP activity have been implicated in cancer cell survival, migration, and invasion, making them attractive targets for anticancer drug development. Similarly, abnormalities in RPTP signaling have been associated with neurodegenerative diseases like Alzheimer’s and Parkinson’s, highlighting their importance in neuronal function and disease pathology.
Small Molecule Drug Design Strategies for RPTPs:
Designing small molecule drugs that selectively target RPTPs poses numerous challenges due to the complex mechanism by which these receptors function. However, advances in our understanding of the structural and functional properties of RPTPs have paved the way for the development of innovative drug design strategies. Some key approaches include:
High-Throughput Screening (HTS):
HTS involves screening large compound libraries to identify molecules that interact with specific RPTPs. Assays measuring changes in enzymatic activity or protein-protein interactions can be employed to identify potential inhibitors or activators of RPTPs. HTS allows the rapid evaluation of a large number of compounds, helping to identify lead molecules for further development.
Rational Design:
Rational drug design utilizes knowledge of the structure-function relationships of RPTPs to design molecules that interact with specific binding sites on the receptors. Structural information, such as X-ray crystallography or molecular modeling, facilitates the identification of binding sites and the design of molecules that fit precisely into these sites. Rational design approaches include structure-based, ligand-based, and fragment-based drug design.
Natural Product Inspired Drug Design:
Natural products have long served as a source of lead compounds for drug development. By studying natural compounds that interact with RPTPs, researchers can gain insights into the structural features necessary for activity. These natural product scaffolds can then be used as starting points for the development of synthetic small molecule drugs with improved properties such as potency, selectivity, and bioavailability.
Fragment-Based Drug Design:
Fragment-based drug design involves screening small, low molecular weight compounds (fragments) for binding to specific target sites on RPTPs. By identifying fragments that bind to different regions of the receptor, medicinal chemists can design larger molecules that combine multiple fragments to create high-affinity and selective RPTP modulators.
Challenges and Future Prospects:
Designing small molecule drugs targeting RPTPs presents several challenges, including selectivity, pharmacokinetics, and delivery to the target site. Achieving selectivity is particularly important to avoid off-target effects. Moreover, as RPTPs are membrane-spanning proteins, developing molecules that can effectively traverse cellular membranes is crucial for reaching their targets.
Future prospects in this field include the development of advanced computational techniques, improved screening technologies, and the exploration of novel chemical space through fragment-based design and natural product-inspired approaches. These advancements, coupled with a deeper understanding of RPTP biology, hold promise for the discovery and development of selective small molecule drugs for the treatment of various diseases.
Conclusion:
Receptor protein tyrosine phosphatases represent valuable therapeutic targets for a range of diseases. Harnessing small molecule drug design strategies, such as rational design, high-throughput screening, natural product-inspired design, and fragment-based approaches, enables researchers to develop selective modulators of RPTP activity. With ongoing advancements and innovative approaches in this field, there is great potential to expand our therapeutic toolkit and develop effective treatments targeting RPTPs, ultimately improving patient outcomes in various disease settings.