PPI (protein-protein interaction) inhibitors tripeptide mimetics are small molecules that mimic the structural features of specific tripeptides involved in protein-protein interactions. These inhibitors are designed to disrupt or modulate PPIs, which play crucial roles in various biological processes and diseases. By mimicking the key binding motifs of the tripeptides involved in the PPIs, these small molecules can interfere with the interaction and potentially provide therapeutic benefits.
Design Principles and Strategies:
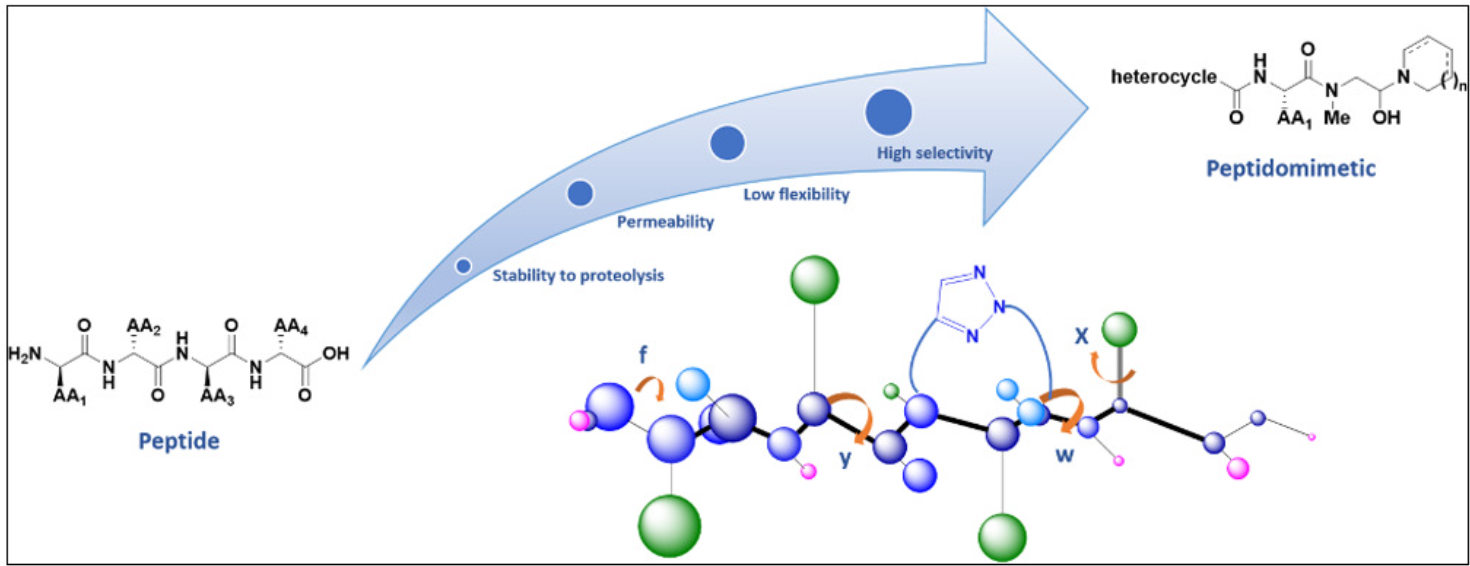
The design of PPI inhibitors tripeptide mimetics involves understanding the structural features, binding modes, and functional groups of the target tripeptides involved in the PPIs. Through rational design and computational approaches, such as molecular docking and molecular dynamics simulations, the mimetics are optimized for improved binding affinity and selectivity. Strategic modifications are made to enhance their stability, cellular permeability, and pharmacokinetic properties.
Structure and Synthetic Diversity:
PPI inhibitors tripeptide mimetics can exhibit diverse structural scaffolds, which can be derived from various peptidomimetic architectures such as lactams, peptoids, beta-turn mimetics, and macrocycles. These scaffolds are modified to resemble the key interactions and conformational features observed in the target tripeptides. The modifications can include substitutions of amino acid residues, introduction of non-natural functionalities, and incorporation of stereochemical constraints.
Synthesis and Screening:
Synthesis of PPI inhibitors tripeptide mimetics generally involves solid-phase or solution-phase techniques, and often employs combinatorial chemistry to generate a library of compounds with structural diversity. High-throughput screening methods, such as fluorescence polarization, surface plasmon resonance, NMR spectroscopy, or X-ray crystallography, are utilized to evaluate the binding affinity, selectivity, and mechanism of action of these molecules. These screening techniques assist in identifying lead compounds with potent inhibitory activity against the target PPI.
Applications and Therapeutic Potential:
PPI inhibitors tripeptide mimetics have shown promise in various therapeutic areas. By disrupting or modulating specific PPIs, these inhibitors can potentially intervene in disease pathways, inhibit aberrant signaling, and restore normal cellular functions. They have been investigated for applications in cancer therapy, where specific PPIs are implicated in oncogenic signaling pathways. Additionally, these mimetics have been explored as potential treatments for viral infections, autoimmune disorders, and neurodegenerative diseases.
Challenges and Future Directions:
Despite their potential, the development of PPI inhibitors tripeptide mimetics faces challenges. Achieving high potency, selectivity, and oral bioavailability while minimizing off-target effects and toxicity remains a key focus. Additionally, identifying appropriate PPIs as therapeutic targets and understanding the complex biology of PPI networks are ongoing challenges. Continued advancements in computational modeling, synthetic chemistry, and screening technologies will be crucial for overcoming these obstacles and advancing the field.
Conclusion:
PPI inhibitors tripeptide mimetics represent a valuable approach in the development of therapeutics targeting protein-protein interactions. By mimicking the binding motifs of tripeptides involved in PPIs, these small molecules offer the potential to disrupt disease-associated protein interactions and provide new strategies for therapeutic intervention. Ongoing research in this area holds immense promise for addressing unmet medical needs and advancing precision medicine approaches.