Abstract:
Covalent inhibitors have emerged as a promising class of therapeutics that offer unique advantages in drug discovery. This article delves into the concept of covalent inhibition and highlights the development and applications of the Covalent Inhibitors Library. By targeting specific disease-associated proteins through irreversible binding, covalent inhibitors provide enhanced potency, selectivity, and prolonged duration of action. We explore the design strategies, synthetic methodologies, and potential therapeutic applications of this versatile library.
Introduction:
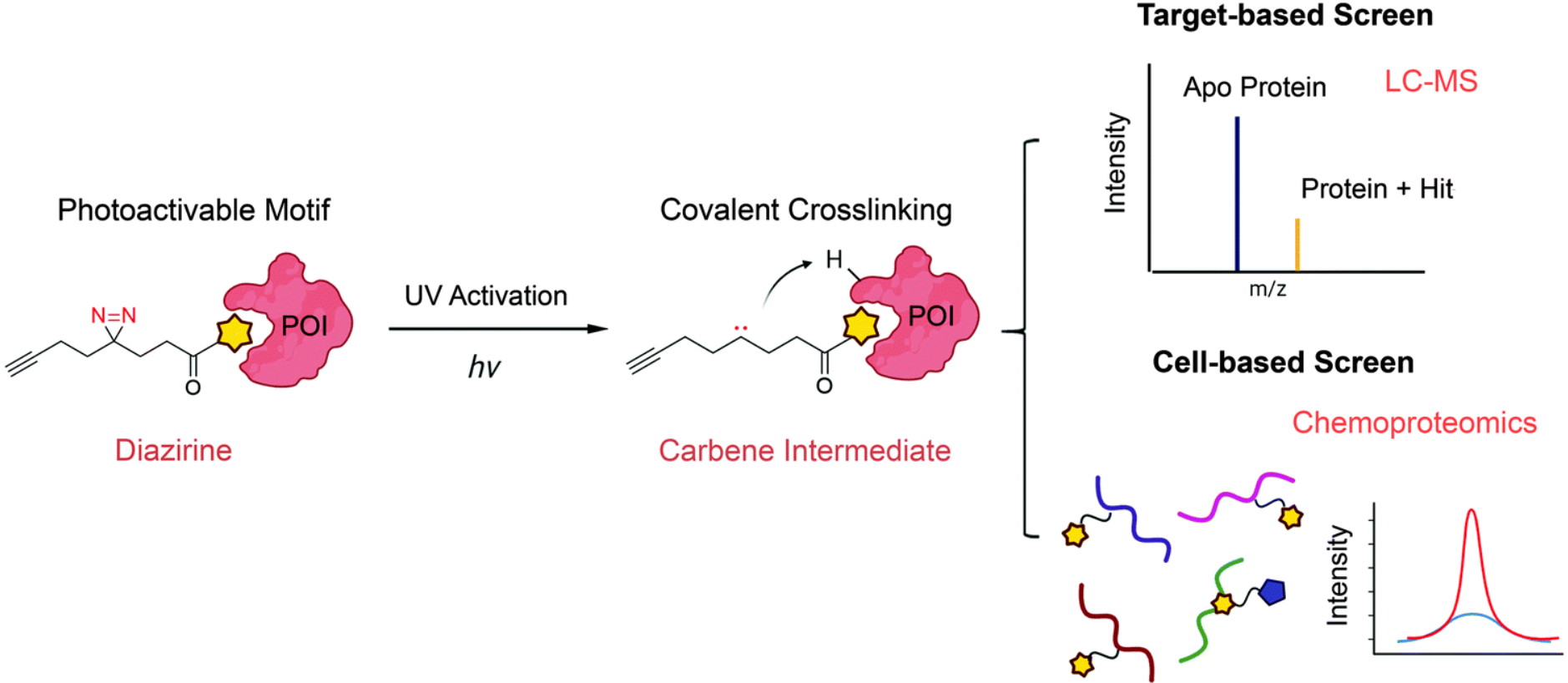
Covalent inhibitors are small molecules that form a stable bond with a target protein via covalent interactions. The formation of a covalent bond between the inhibitor and target protein enables irreversible inhibition, leading to increased potency and prolonged target engagement compared to reversible inhibitors. The Covalent Inhibitors Library enables researchers to harness the power of this unique mode of action to develop novel therapeutics for various diseases.
Design and Synthesis of Covalent Inhibitors:
The creation of the Covalent Inhibitors Library involves a multi-step process, starting with the identification of suitable target proteins. Once the protein target is selected, computational tools are employed to design covalent inhibitors by identifying specific functional groups capable of forming a stable covalent bond with the target protein’s reactive residue. Synthetic methodologies, including medicinal chemistry techniques and innovative synthetic routes, are then employed to generate a diverse array of covalent inhibitors with varying chemical scaffolds and optimized drug-like properties.
Advantages and Challenges of Covalent Inhibition:
Covalent inhibitors offer several advantages over reversible inhibitors. By irreversibly binding to the target protein, covalent inhibitors achieve enhanced potency due to a prolonged duration of target inhibition. This prolonged target engagement is particularly advantageous for proteins with a high turnover rate or for disease-associated proteins that require continuous suppression. Additionally, the irreversible nature of covalent inhibitors provides increased selectivity, as the covalent bond formation requires a specific reactive residue in the target protein.
However, the development of covalent inhibitors also presents challenges. Off-target binding and potential toxicity can occur if the selectivity of the covalent inhibitor is not carefully optimized. Moreover, designing covalent inhibitors requires a deep understanding of the target protein and the appropriate reactive residue to target. Careful consideration must be given to the stability of the covalent bond formed and the irreversible modification’s consequences on the target protein’s function.
Therapeutic Applications and Future Directions:
The Covalent Inhibitors Library holds immense potential in drug discovery for a wide range of diseases. Covalent inhibitors have demonstrated clinical efficacy against various targets, including kinases, proteases, and other disease-associated proteins. They have shown promise in treating cancer, inflammatory disorders, infectious diseases, and neurological conditions. With ongoing advancements in synthetic methodologies and target identification, the Covalent Inhibitors Library opens new avenues for developing next-generation therapeutics.
Conclusion:
The Covalent Inhibitors Library represents a transformative approach in drug discovery, harnessing the power of irreversible binding to target disease-associated proteins. Covalent inhibitors offer unique advantages, including enhanced potency, prolonged duration of action, and increased selectivity. While challenges exist, the growth and success of covalent inhibitors underline their potential in various therapeutic areas. As research continues, the Covalent Inhibitors Library holds tremendous promise for revolutionizing the development of novel therapeutics and advancing precision medicine.