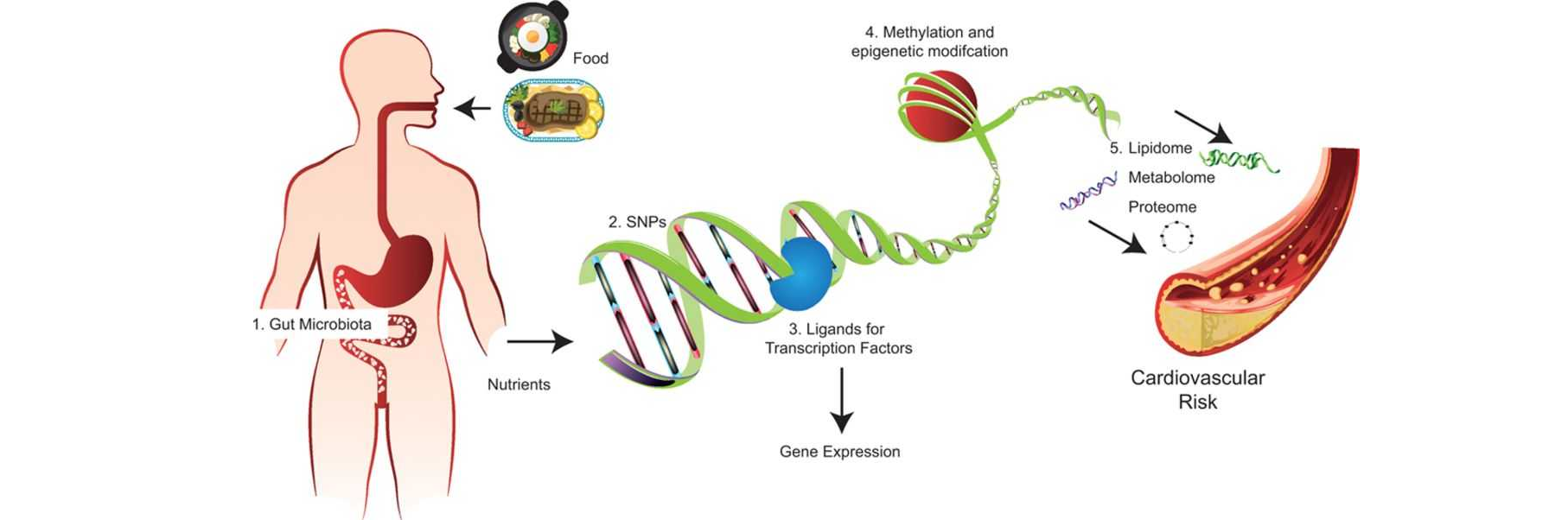
Introduction:
Epigenetics, the study of heritable changes in gene expression without alterations to the underlying DNA sequence, holds immense promise in unraveling the complexity of various diseases. In recent years, compounds targeting epigenetic mechanisms have emerged as valuable tools in understanding, modifying, and potentially treating these diseases. This blog highlights the significance of compounds targeting epigenetics and explores their potential in unlocking new therapeutic avenues.
Understanding Epigenetic Mechanisms:
Epigenetic modifications, including DNA methylation, histone modifications, and non-coding RNA molecules, play a crucial role in regulating gene expression patterns. Dysregulation of these mechanisms can contribute to the development and progression of a range of diseases, such as cancer, neurodegenerative disorders, and autoimmune conditions. By targeting epigenetic processes, compounds have the potential to modulate gene expression, potentially reversing disease-associated epigenetic alterations.
Compounds Targeting DNA Methylation:
One of the most extensively studied epigenetic modifications is DNA methylation. DNA methyltransferase inhibitors, such as 5-azacytidine and 5-aza-2′-deoxycytidine (decitabine), offer a means to reverse aberrant DNA methylation patterns. These compounds can reactivate silenced genes and restore normal gene expression, making them promising candidates for the treatment of certain cancers, including myelodysplastic syndromes and acute myeloid leukemia.
Histone Modifying Compounds:
Histone proteins play a crucial role in DNA packaging and gene regulation. Several compounds designed to target histone modifications have been developed, including histone deacetylase (HDAC) inhibitors and histone methyltransferase inhibitors. HDAC inhibitors, such as vorinostat and romidepsin, can interfere with the removal of acetyl groups from histones, leading to altered gene expression patterns. These compounds show promise in the treatment of various cancers, including cutaneous T-cell lymphoma and multiple myeloma.
Epigenetic Compounds in Cancer Therapies:
The field of epigenetic therapeutics has shown significant potential in cancer treatment. The FDA-approved drug, vorinostat, serves as a prime example, demonstrating improved outcomes in patients with cutaneous T-cell lymphoma. Combination therapies that involve epigenetic compounds, such as DNA methyltransferase inhibitors and HDAC inhibitors, are being explored to enhance treatment efficacy and overcome drug resistance mechanisms.
Emerging Applications in Neurodegenerative Disorders:
Epigenetic modifications have also emerged as promising targets for neurodegenerative disorders, including Alzheimer’s disease and Parkinson’s disease. Compounds targeting DNA methylation and histone modifications show potential in modifying key disease-associated pathways and regulating gene expression patterns. While still in early stages, these compounds represent a promising avenue for developing disease-modifying treatments for neurodegenerative conditions.
Challenges and Future Directions:
Despite progress made in the field of compounds targeting epigenetics, challenges remain. Optimizing the selectivity, stability, and delivery of these compounds is crucial to minimize off-target effects and maximize therapeutic efficacy. Further research into disease-specific epigenetic alterations and the development of targeted therapies are essential for maximizing the potential of these compounds in clinical practice.
Conclusion:
Compounds targeting epigenetic mechanisms have revolutionized our understanding of gene regulation and offer new possibilities for therapeutic interventions. By modifying epigenetic marks, these compounds can potentially reverse disease-associated alterations, offering hope for the treatment of a wide range of diseases, including cancer and neurodegenerative disorders. Continued research and clinical trials are needed to further harness the potential of these compounds and pave the way for personalized epigenetic-based therapies in the future.